
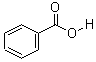
CAS: 65-85-0
Benzoic acid
Benzoic acid
Properties:
- Product Name: Benzoic acid
- Synonyms:3246: Melting point standard benzoic acid; Benzoic-12C7 acid, 13C-depleted; Benzoic acid, USP Grade; 4-Carboxypolystyrene; benzoate; Industrial-use benzoic acid
- CAS RN.: 65-85-0
- EINECS: 200-618-2
- Molecular Weight: 122.1224
- Molecular Formula: C7H6O2
- Melting Point(℃): 121-123℃
- Boiling Point(℃): 249.3°C at 760 mmHg
- Flash Point(℃): 111.4°C
- Water Solubility: Slightly soluble. 0.34 g/100 mL
- Hazard Symbols: Xn Details
- Xn:
- Risk Codes: R22;R36/37/38;R42/43; Details
- R22;R36/37/38;R42/43;: Details
- Safety Description: S22;S24;S26;S37/39;S45;Details
- S22;S24;S26;S37/39;S45;: Details
FAQ:
What is benzoic acid and how is it used in various industries?
Benzoic acid is a white, crystalline organic compound that is widely used as a food preservative, as well as in the manufacturing of various products in industries such as pharmaceuticals, cosmetics, and plastics. Its antimicrobial properties make it an effective preservative in food products, while its ability to react with other chemicals makes it versatile in industrial processes.
How does benzoic acid act as a food preservative?
Benzoic acid inhibits the growth of bacteria and fungi by lowering the pH of food products, creating an environment that is unfavorable for microbial growth. This preservation method helps extend the shelf life of food products and prevents them from spoiling.
What are the benefits of using benzoic acid in cosmetic products?
In cosmetic products, benzoic acid acts as a preservative to prevent the growth of harmful microorganisms, ensuring the safety and stability of these products. It is commonly used in lotions, creams, and shampoos to maintain their quality and extend their shelf life.
How is benzoic acid used in pharmaceuticals?
In the pharmaceutical industry, benzoic acid is utilized as a precursor in the synthesis of various drugs and active pharmaceutical ingredients. It is also used as a preservative in liquid medications to prevent microbial contamination and maintain their efficacy.
What are the environmental implications of benzoic acid usage?
While benzoic acid is considered safe for use in various industries, it is important to handle and dispose of it properly to minimize its environmental impact. Proper storage, handling, and disposal practices are essential to prevent contamination of water sources and ecosystems.
Benzoic acid is a white, crystalline organic compound that is widely used as a food preservative, as well as in the manufacturing of various products in industries such as pharmaceuticals, cosmetics, and plastics. Its antimicrobial properties make it an effective preservative in food products, while its ability to react with other chemicals makes it versatile in industrial processes.
How does benzoic acid act as a food preservative?
Benzoic acid inhibits the growth of bacteria and fungi by lowering the pH of food products, creating an environment that is unfavorable for microbial growth. This preservation method helps extend the shelf life of food products and prevents them from spoiling.
What are the benefits of using benzoic acid in cosmetic products?
In cosmetic products, benzoic acid acts as a preservative to prevent the growth of harmful microorganisms, ensuring the safety and stability of these products. It is commonly used in lotions, creams, and shampoos to maintain their quality and extend their shelf life.
How is benzoic acid used in pharmaceuticals?
In the pharmaceutical industry, benzoic acid is utilized as a precursor in the synthesis of various drugs and active pharmaceutical ingredients. It is also used as a preservative in liquid medications to prevent microbial contamination and maintain their efficacy.
What are the environmental implications of benzoic acid usage?
While benzoic acid is considered safe for use in various industries, it is important to handle and dispose of it properly to minimize its environmental impact. Proper storage, handling, and disposal practices are essential to prevent contamination of water sources and ecosystems.

