
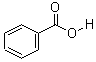
CAS: 65-85-0
Melting point standard benzoic acid
Melting point standard benzoic acid
Properties:
- Product Name: Benzoic acid
- Synonyms:3246: Melting point standard benzoic acid; Benzoic-12C7 acid, 13C-depleted; Benzoic acid, USP Grade; 4-Carboxypolystyrene; benzoate; Industrial-use benzoic acid
- CAS RN.: 65-85-0
- EINECS: 200-618-2
- Molecular Weight: 122.1224
- Molecular Formula: C7H6O2
- Melting Point(℃): 121-123℃
- Boiling Point(℃): 249.3°C at 760 mmHg
- Flash Point(℃): 111.4°C
- Water Solubility: Slightly soluble. 0.34 g/100 mL
- Hazard Symbols: Xn Details
- Xn:
- Risk Codes: R22;R36/37/38;R42/43; Details
- R22;R36/37/38;R42/43;: Details
- Safety Description: S22;S24;S26;S37/39;S45;Details
- S22;S24;S26;S37/39;S45;: Details
FAQ:
What is a melting point standard and why is benzoic acid used as a standard for melting
point determination?
Benzoic acid is a common standard substance used in the determination of melting points for organic compounds. Its melting point is well-known and consistent, making it ideal for calibration purposes. By using benzoic acid as a standard, chemists can ensure the accuracy and reliability of their melting point measurements.
How does the melting point standard benzoic acid work in practice?
In practice, benzoic acid is mixed with the sample compound being tested. The mixture is then heated gradually until both substances melt. The temperature at which the mixture begins to melt and the temperature range over which it melts completely is recorded as the melting point. By comparing these measurements to the known melting point of benzoic acid, chemists can verify the accuracy of their results.
What are the benefits of using benzoic acid as a melting point standard?
One of the main benefits of using benzoic acid as a melting point standard is its high level of purity and consistency. Benzoic acid is readily available in pure form, ensuring that any impurities in the sample being tested do not affect the accuracy of the melting point determination. Additionally, its well-defined melting point allows for precise calibration of melting point apparatuses.
How does the use of a melting point standard like benzoic acid contribute to the field of organic chemistry?
In organic chemistry, the determination of melting points is a critical step in the identification and characterization of organic compounds. By using a standard substance like benzoic acid, chemists can ensure the reliability and reproducibility of their results. This is especially important in fields such as pharmaceuticals, where the purity and identity of compounds are crucial to their efficacy.
What factors should be considered when using benzoic acid as a melting point standard?
When using benzoic acid as a melting point standard, it is important to ensure that the substance is pure and free from any contaminants. Additionally, proper calibration of the melting point apparatus and accurate temperature measurement are essential for obtaining reliable results. By taking these factors into account, chemists can confidently use benzoic acid as a standard for their melting point determinations.
Benzoic acid is a common standard substance used in the determination of melting points for organic compounds. Its melting point is well-known and consistent, making it ideal for calibration purposes. By using benzoic acid as a standard, chemists can ensure the accuracy and reliability of their melting point measurements.
How does the melting point standard benzoic acid work in practice?
In practice, benzoic acid is mixed with the sample compound being tested. The mixture is then heated gradually until both substances melt. The temperature at which the mixture begins to melt and the temperature range over which it melts completely is recorded as the melting point. By comparing these measurements to the known melting point of benzoic acid, chemists can verify the accuracy of their results.
What are the benefits of using benzoic acid as a melting point standard?
One of the main benefits of using benzoic acid as a melting point standard is its high level of purity and consistency. Benzoic acid is readily available in pure form, ensuring that any impurities in the sample being tested do not affect the accuracy of the melting point determination. Additionally, its well-defined melting point allows for precise calibration of melting point apparatuses.
How does the use of a melting point standard like benzoic acid contribute to the field of organic chemistry?
In organic chemistry, the determination of melting points is a critical step in the identification and characterization of organic compounds. By using a standard substance like benzoic acid, chemists can ensure the reliability and reproducibility of their results. This is especially important in fields such as pharmaceuticals, where the purity and identity of compounds are crucial to their efficacy.
What factors should be considered when using benzoic acid as a melting point standard?
When using benzoic acid as a melting point standard, it is important to ensure that the substance is pure and free from any contaminants. Additionally, proper calibration of the melting point apparatus and accurate temperature measurement are essential for obtaining reliable results. By taking these factors into account, chemists can confidently use benzoic acid as a standard for their melting point determinations.

