
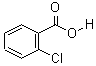
CAS: 118-91-2
Ortho-chlorobenzoic Acid
Ortho-chlorobenzoic Acid
Properties:
- Product Name: 2-Chlorobenzoic acid
- Synonyms:4058: 2-CBA; o-Chlorobenzoic acid; nickel(2+) bis(2-chlorobenzoate); 2-chlorobenzoate; Ortho-chlorobenzoic Acid
- CAS RN.: 118-91-2
- EINECS: 204-285-4
- Molecular Weight: 156.5685
- Molecular Formula: C7H5ClO2
- Melting Point(℃): 139-143℃
- Boiling Point(℃): 275.7°C at 760 mmHg
- Flash Point(℃): 120.5°C
- Water Solubility: soluble in hot water
- Hazard Symbols: Xi Details
- Xi:
- Risk Codes: R36/37/38; Details
- R36/37/38;: Details
- Safety Description: S26;S37/39;Details
- S26;S37/39;: Details
FAQ:
What is ortho-chlorobenzoic acid and how is it used?
Ortho-chlorobenzoic acid, also known as 2-chlorobenzoic acid, is a chemical compound with the formula C7H5ClO2. It is a white crystal that is soluble in various solvents, such as ethanol and chloroform. This compound is commonly used as an intermediate in the synthesis of pharmaceuticals, dyes, and pesticides.
How is ortho-chlorobenzoic acid synthesized?
Ortho-chlorobenzoic acid can be synthesized through the chlorination of benzoic acid using a chlorine source such as phosphorus pentachloride (PCl5). The reaction typically takes place under anhydrous conditions and the product can be isolated through crystallization.
What are some of the applications of ortho-chlorobenzoic acid?
Ortho-chlorobenzoic acid is used as an intermediate in the synthesis of various pharmaceuticals, including anti-inflammatory drugs and antipsychotics. Additionally, it is employed in the production of dyes for the textile industry and as a starting material for the synthesis of pesticides.
What are the potential hazards associated with ortho-chlorobenzoic acid?
Ortho-chlorobenzoic acid should be handled with care as it is a corrosive substance that can cause skin and eye irritation. Inhalation of its vapors may also lead to respiratory irritation. Proper personal protective equipment, such as gloves and goggles, should be worn when working with this compound.
Are there any regulatory restrictions on the use of ortho-chlorobenzoic acid?
Ortho-chlorobenzoic acid is a regulated substance due to its potential health hazards. Users should consult local regulations and guidelines regarding its handling, storage, and disposal. It is important to follow proper safety protocols to minimize the risks associated with this chemical compound.
Ortho-chlorobenzoic acid, also known as 2-chlorobenzoic acid, is a chemical compound with the formula C7H5ClO2. It is a white crystal that is soluble in various solvents, such as ethanol and chloroform. This compound is commonly used as an intermediate in the synthesis of pharmaceuticals, dyes, and pesticides.
How is ortho-chlorobenzoic acid synthesized?
Ortho-chlorobenzoic acid can be synthesized through the chlorination of benzoic acid using a chlorine source such as phosphorus pentachloride (PCl5). The reaction typically takes place under anhydrous conditions and the product can be isolated through crystallization.
What are some of the applications of ortho-chlorobenzoic acid?
Ortho-chlorobenzoic acid is used as an intermediate in the synthesis of various pharmaceuticals, including anti-inflammatory drugs and antipsychotics. Additionally, it is employed in the production of dyes for the textile industry and as a starting material for the synthesis of pesticides.
What are the potential hazards associated with ortho-chlorobenzoic acid?
Ortho-chlorobenzoic acid should be handled with care as it is a corrosive substance that can cause skin and eye irritation. Inhalation of its vapors may also lead to respiratory irritation. Proper personal protective equipment, such as gloves and goggles, should be worn when working with this compound.
Are there any regulatory restrictions on the use of ortho-chlorobenzoic acid?
Ortho-chlorobenzoic acid is a regulated substance due to its potential health hazards. Users should consult local regulations and guidelines regarding its handling, storage, and disposal. It is important to follow proper safety protocols to minimize the risks associated with this chemical compound.

