
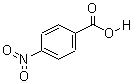
CAS: 62-23-7
P-Nitrobenzoic acid
P-Nitrobenzoic acid
Properties:
- Product Name: 4-Nitrobenzoic acid
- Synonyms:2231: P-Nitrobenzoic acid; 4-nitrobenzoate; Para Nitrobenzoic acid; PNBA
- CAS RN.: 62-23-7
- EINECS: 200-526-2
- Molecular Weight: 166.1115
- Molecular Formula: C7H4NO4
- Melting Point(℃): 239-242℃
- Boiling Point(℃): 359.1°C at 760 mmHg
- Flash Point(℃): 166.5°C
- Water Solubility:
- Hazard Symbols: Xn Details
- Xn:
- Risk Codes: R22; Details
- R22;: Details
- Safety Description: S24/25;Details
- S24/25;: Details
FAQ:
What is P-Nitrobenzoic acid and what are its uses?
P-Nitrobenzoic acid, also known as 4-nitrobenzoic acid, is a chemical compound with the formula C7H5NO4. It is a yellow crystalline solid that is primarily used as a building block in the synthesis of various organic compounds. It is commonly used in research laboratories and industrial settings as a precursor in the preparation of dyes, pharmaceuticals, and other organic compounds.
How is P-Nitrobenzoic acid synthesized?
P-Nitrobenzoic acid can be synthesized through the nitration of benzoic acid with a mixture of concentrated nitric and sulfuric acids. The nitration reaction involves the substitution of a hydrogen atom on the benzene ring of benzoic acid with a nitro group (-NO2) to form P-Nitrobenzoic acid. The reaction typically takes place under controlled conditions to ensure a high yield of the desired product.
What are the physical properties of P-Nitrobenzoic acid?
P-Nitrobenzoic acid is a yellow crystalline solid with a melting point of around 237-238 degrees Celsius. It is sparingly soluble in water but soluble in organic solvents such as ethanol and acetone. The compound has a molecular weight of 181.12 g/mol and a molecular formula of C7H5NO4. It is important to handle P-Nitrobenzoic acid with caution as it may be toxic if ingested or inhaled.
What are some common applications of P-Nitrobenzoic acid?
P-Nitrobenzoic acid is commonly used as a precursor in the synthesis of various organic compounds due to its versatile reactivity. It is often utilized in the preparation of dyes, pharmaceuticals, and other specialty chemicals. In addition, P-Nitrobenzoic acid can be used as a reagent in organic chemistry reactions such as esterification, amidation, and peptide synthesis. Its ability to undergo various transformations makes it a valuable building block in organic synthesis.
Is P-Nitrobenzoic acid considered hazardous or toxic?
P-Nitrobenzoic acid is classified as a harmful and toxic substance that should be handled with caution. It may cause irritation to the skin, eyes, and respiratory tract upon contact or inhalation. It is important to use appropriate personal protective equipment such as gloves, goggles, and a lab coat when working with P-Nitrobenzoic acid. In addition, the compound should be stored in a cool, dry place away from heat sources and incompatible materials to prevent accidents or chemical reactions.
P-Nitrobenzoic acid, also known as 4-nitrobenzoic acid, is a chemical compound with the formula C7H5NO4. It is a yellow crystalline solid that is primarily used as a building block in the synthesis of various organic compounds. It is commonly used in research laboratories and industrial settings as a precursor in the preparation of dyes, pharmaceuticals, and other organic compounds.
How is P-Nitrobenzoic acid synthesized?
P-Nitrobenzoic acid can be synthesized through the nitration of benzoic acid with a mixture of concentrated nitric and sulfuric acids. The nitration reaction involves the substitution of a hydrogen atom on the benzene ring of benzoic acid with a nitro group (-NO2) to form P-Nitrobenzoic acid. The reaction typically takes place under controlled conditions to ensure a high yield of the desired product.
What are the physical properties of P-Nitrobenzoic acid?
P-Nitrobenzoic acid is a yellow crystalline solid with a melting point of around 237-238 degrees Celsius. It is sparingly soluble in water but soluble in organic solvents such as ethanol and acetone. The compound has a molecular weight of 181.12 g/mol and a molecular formula of C7H5NO4. It is important to handle P-Nitrobenzoic acid with caution as it may be toxic if ingested or inhaled.
What are some common applications of P-Nitrobenzoic acid?
P-Nitrobenzoic acid is commonly used as a precursor in the synthesis of various organic compounds due to its versatile reactivity. It is often utilized in the preparation of dyes, pharmaceuticals, and other specialty chemicals. In addition, P-Nitrobenzoic acid can be used as a reagent in organic chemistry reactions such as esterification, amidation, and peptide synthesis. Its ability to undergo various transformations makes it a valuable building block in organic synthesis.
Is P-Nitrobenzoic acid considered hazardous or toxic?
P-Nitrobenzoic acid is classified as a harmful and toxic substance that should be handled with caution. It may cause irritation to the skin, eyes, and respiratory tract upon contact or inhalation. It is important to use appropriate personal protective equipment such as gloves, goggles, and a lab coat when working with P-Nitrobenzoic acid. In addition, the compound should be stored in a cool, dry place away from heat sources and incompatible materials to prevent accidents or chemical reactions.

