
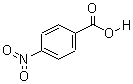
CAS: 62-23-7
Para Nitrobenzoic acid
Para Nitrobenzoic acid
Properties:
- Product Name: 4-Nitrobenzoic acid
- Synonyms:2231: P-Nitrobenzoic acid; 4-nitrobenzoate; Para Nitrobenzoic acid; PNBA
- CAS RN.: 62-23-7
- EINECS: 200-526-2
- Molecular Weight: 166.1115
- Molecular Formula: C7H4NO4
- Melting Point(℃): 239-242℃
- Boiling Point(℃): 359.1°C at 760 mmHg
- Flash Point(℃): 166.5°C
- Water Solubility:
- Hazard Symbols: Xn Details
- Xn:
- Risk Codes: R22; Details
- R22;: Details
- Safety Description: S24/25;Details
- S24/25;: Details
FAQ:
What is para-Nitrobenzoic acid and what are its uses?
para-Nitrobenzoic acid, also known as 4-nitrobenzoic acid, is a chemical compound with the formula C7H5NO4. It is a yellow crystalline solid that is commonly used as an intermediate in the synthesis of pharmaceuticals, dyes, and polymers. para-Nitrobenzoic acid is also utilized in the production of UV absorbers and organic light-emitting diodes.
How is para-Nitrobenzoic acid synthesized?
para-Nitrobenzoic acid can be synthesized through the nitration of benzoic acid with a mixture of nitric and sulfuric acids. The nitration process involves the substitution of a hydrogen atom on the benzene ring of benzoic acid with a nitro group (-NO2), resulting in the formation of para-nitrobenzoic acid.
What are the key properties of para-Nitrobenzoic acid?
para-Nitrobenzoic acid is a yellow crystalline solid with a melting point of 242-245°C. It is sparingly soluble in water but soluble in organic solvents such as ethanol, methanol, and acetone. The compound exhibits strong acidic properties due to the presence of the carboxylic acid group (-COOH), making it a versatile building block for organic synthesis.
What are the safety considerations when handling para-Nitrobenzoic acid?
When handling para-Nitrobenzoic acid, it is important to wear appropriate personal protective equipment, including gloves, safety goggles, and a lab coat. The compound should be stored in a cool, dry place away from direct sunlight and incompatible materials. Avoid inhalation and skin contact with para-Nitrobenzoic acid, as it may cause irritation or allergic reactions.
What are some of the applications of para-Nitrobenzoic acid in the pharmaceutical industry?
para-Nitrobenzoic acid is widely used in the pharmaceutical industry as a key intermediate in the synthesis of various drugs and active pharmaceutical ingredients (APIs). It serves as a building block for the production of analgesics, antipyretics, antimicrobials, and anticancer agents. Additionally, para-Nitrobenzoic acid is utilized in the development of drug delivery systems and prodrugs for improved bioavailability and therapeutic efficacy.
para-Nitrobenzoic acid, also known as 4-nitrobenzoic acid, is a chemical compound with the formula C7H5NO4. It is a yellow crystalline solid that is commonly used as an intermediate in the synthesis of pharmaceuticals, dyes, and polymers. para-Nitrobenzoic acid is also utilized in the production of UV absorbers and organic light-emitting diodes.
How is para-Nitrobenzoic acid synthesized?
para-Nitrobenzoic acid can be synthesized through the nitration of benzoic acid with a mixture of nitric and sulfuric acids. The nitration process involves the substitution of a hydrogen atom on the benzene ring of benzoic acid with a nitro group (-NO2), resulting in the formation of para-nitrobenzoic acid.
What are the key properties of para-Nitrobenzoic acid?
para-Nitrobenzoic acid is a yellow crystalline solid with a melting point of 242-245°C. It is sparingly soluble in water but soluble in organic solvents such as ethanol, methanol, and acetone. The compound exhibits strong acidic properties due to the presence of the carboxylic acid group (-COOH), making it a versatile building block for organic synthesis.
What are the safety considerations when handling para-Nitrobenzoic acid?
When handling para-Nitrobenzoic acid, it is important to wear appropriate personal protective equipment, including gloves, safety goggles, and a lab coat. The compound should be stored in a cool, dry place away from direct sunlight and incompatible materials. Avoid inhalation and skin contact with para-Nitrobenzoic acid, as it may cause irritation or allergic reactions.
What are some of the applications of para-Nitrobenzoic acid in the pharmaceutical industry?
para-Nitrobenzoic acid is widely used in the pharmaceutical industry as a key intermediate in the synthesis of various drugs and active pharmaceutical ingredients (APIs). It serves as a building block for the production of analgesics, antipyretics, antimicrobials, and anticancer agents. Additionally, para-Nitrobenzoic acid is utilized in the development of drug delivery systems and prodrugs for improved bioavailability and therapeutic efficacy.

