
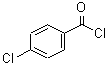
CAS: 122-01-0
4-Chlorobenzoyl chloride
4-Chlorobenzoyl chloride
Properties:
- Product Name: 4-Chlorobenzoyl chloride
- Synonyms:3331: PCOC; p-Chlorobenzolychloride; 4-Chlorobenzolychloride; p-chlorlbenzoyl chloride; p-chlorobenzoyl chloride; parachlorobenzoyl chloride; 4-chloro-6-methylquinazoline; Benzoyl chloride, 4-chloro; Benzoyl chloride, p-chloro
- CAS RN.: 122-01-0
- EINECS: 204-515-3
- Molecular Weight: 175.0141
- Molecular Formula: C4H4Cl2O
- Density: 1.292g/cm3
- Melting Point(℃): 12-14℃
- Boiling Point(℃): 298.2°C at 760 mmHg
- Flash Point(℃): 162.3°C
- refractive_index: 1.643
- Hazard Symbols: C Details
- C:
- Risk Codes: R34; Details
- R34;: Details
- Safety Description: S26;S28A;Details
- S26;S28A;: Details
FAQ:
What is 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is a chemical compound that is commonly used as an intermediate in the production of various pharmaceuticals, dyes, and agrochemicals. It is a white to pale yellow solid with a pungent odor.
What are the key applications of 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is primarily used in the synthesis of pharmaceutical compounds such as benzophenones, benzoyl benzoates, and aromatic esters. It is also utilized in the manufacturing of dyes, pesticides, and other fine chemicals.
How is 4-Chlorobenzoyl chloride typically synthesized?
4-Chlorobenzoyl chloride is usually prepared by the reaction of 4-chlorobenzoic acid with thionyl chloride or phosphorus pentachloride. This process yields a high purity product with good yield.
What are the key properties of 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is a highly reactive compound that is sensitive to moisture and air. It has a melting point of around 15-20°C and a boiling point of 121-123°C. It is soluble in a variety of organic solvents such as dichloromethane, ether, and chloroform.
What are the safety considerations when handling 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is a corrosive compound that can cause skin and eye irritation upon contact. It is important to handle it with proper safety precautions, including the use of gloves, goggles, and a lab coat. It should also be stored in a cool, dry place away from sources of heat and moisture.
4-Chlorobenzoyl chloride is a chemical compound that is commonly used as an intermediate in the production of various pharmaceuticals, dyes, and agrochemicals. It is a white to pale yellow solid with a pungent odor.
What are the key applications of 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is primarily used in the synthesis of pharmaceutical compounds such as benzophenones, benzoyl benzoates, and aromatic esters. It is also utilized in the manufacturing of dyes, pesticides, and other fine chemicals.
How is 4-Chlorobenzoyl chloride typically synthesized?
4-Chlorobenzoyl chloride is usually prepared by the reaction of 4-chlorobenzoic acid with thionyl chloride or phosphorus pentachloride. This process yields a high purity product with good yield.
What are the key properties of 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is a highly reactive compound that is sensitive to moisture and air. It has a melting point of around 15-20°C and a boiling point of 121-123°C. It is soluble in a variety of organic solvents such as dichloromethane, ether, and chloroform.
What are the safety considerations when handling 4-Chlorobenzoyl chloride?
4-Chlorobenzoyl chloride is a corrosive compound that can cause skin and eye irritation upon contact. It is important to handle it with proper safety precautions, including the use of gloves, goggles, and a lab coat. It should also be stored in a cool, dry place away from sources of heat and moisture.

