
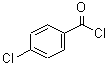
CAS: 122-01-0
Benzoyl chloride, p-chloro
Benzoyl chloride, p-chloro
Properties:
- Product Name: 4-Chlorobenzoyl chloride
- Synonyms:3331: PCOC; p-Chlorobenzolychloride; 4-Chlorobenzolychloride; p-chlorlbenzoyl chloride; p-chlorobenzoyl chloride; parachlorobenzoyl chloride; 4-chloro-6-methylquinazoline; Benzoyl chloride, 4-chloro; Benzoyl chloride, p-chloro
- CAS RN.: 122-01-0
- EINECS: 204-515-3
- Molecular Weight: 175.0141
- Molecular Formula: C4H4Cl2O
- Density: 1.292g/cm3
- Melting Point(℃): 12-14℃
- Boiling Point(℃): 298.2°C at 760 mmHg
- Flash Point(℃): 162.3°C
- refractive_index: 1.643
- Hazard Symbols: C Details
- C:
- Risk Codes: R34; Details
- R34;: Details
- Safety Description: S26;S28A;Details
- S26;S28A;: Details
FAQ:
What is benzoyl chloride, p-chloro?
Benzoyl chloride, p-chloro, is a chemical compound that is commonly used in organic synthesis and chemical processes. It is a colorless to pale yellow liquid with a strong odor, and it is highly reactive due to the presence of a reactive benzoyl group and a chlorine atom.
How is benzoyl chloride, p-chloro used in the industry?
Benzoyl chloride, p-chloro is commonly used as a reagent in organic synthesis to introduce the benzoyl group into various compounds. It is also used in the production of dyes, perfumes, and pharmaceuticals. Additionally, benzoyl chloride, p-chloro is used as a crosslinking agent in the production of polymers and resins.
What are the safety precautions to be taken when working with benzoyl chloride, p-chloro?
When working with benzoyl chloride, p-chloro, it is important to wear appropriate personal protective equipment, including gloves, goggles, and a lab coat. Benzoyl chloride, p-chloro is corrosive and can cause skin and eye irritation, so it is essential to handle it in a well-ventilated area and avoid inhalation of vapors. In case of contact with skin or eyes, rinse thoroughly with water and seek medical attention if necessary.
Are there any specific storage requirements for benzoyl chloride, p-chloro?
Benzoyl chloride, p-chloro should be stored in a cool, dry place away from heat, flames, and incompatible materials. It should be kept in a tightly closed container to prevent exposure to air and moisture, which can cause degradation of the compound. Additionally, benzoyl chloride, p-chloro should be stored away from direct sunlight to avoid decomposition.
What are some common reactions that benzoyl chloride, p-chloro is used in?
Benzoyl chloride, p-chloro is commonly used in the Friedel-Crafts acylation reaction to introduce the benzoyl group into aromatic compounds. It is also used in the preparation of benzophenone, benzoic acid, and various benzoyl derivatives. Additionally, benzoyl chloride, p-chloro can be used in the synthesis of pharmaceutical intermediates and agrochemicals.
Benzoyl chloride, p-chloro, is a chemical compound that is commonly used in organic synthesis and chemical processes. It is a colorless to pale yellow liquid with a strong odor, and it is highly reactive due to the presence of a reactive benzoyl group and a chlorine atom.
How is benzoyl chloride, p-chloro used in the industry?
Benzoyl chloride, p-chloro is commonly used as a reagent in organic synthesis to introduce the benzoyl group into various compounds. It is also used in the production of dyes, perfumes, and pharmaceuticals. Additionally, benzoyl chloride, p-chloro is used as a crosslinking agent in the production of polymers and resins.
What are the safety precautions to be taken when working with benzoyl chloride, p-chloro?
When working with benzoyl chloride, p-chloro, it is important to wear appropriate personal protective equipment, including gloves, goggles, and a lab coat. Benzoyl chloride, p-chloro is corrosive and can cause skin and eye irritation, so it is essential to handle it in a well-ventilated area and avoid inhalation of vapors. In case of contact with skin or eyes, rinse thoroughly with water and seek medical attention if necessary.
Are there any specific storage requirements for benzoyl chloride, p-chloro?
Benzoyl chloride, p-chloro should be stored in a cool, dry place away from heat, flames, and incompatible materials. It should be kept in a tightly closed container to prevent exposure to air and moisture, which can cause degradation of the compound. Additionally, benzoyl chloride, p-chloro should be stored away from direct sunlight to avoid decomposition.
What are some common reactions that benzoyl chloride, p-chloro is used in?
Benzoyl chloride, p-chloro is commonly used in the Friedel-Crafts acylation reaction to introduce the benzoyl group into aromatic compounds. It is also used in the preparation of benzophenone, benzoic acid, and various benzoyl derivatives. Additionally, benzoyl chloride, p-chloro can be used in the synthesis of pharmaceutical intermediates and agrochemicals.

