
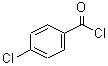
CAS: 122-01-0
p-chlorobenzoyl chloride
p-chlorobenzoyl chloride
Properties:
- Product Name: 4-Chlorobenzoyl chloride
- Synonyms:3331: PCOC; p-Chlorobenzolychloride; 4-Chlorobenzolychloride; p-chlorlbenzoyl chloride; p-chlorobenzoyl chloride; parachlorobenzoyl chloride; 4-chloro-6-methylquinazoline; Benzoyl chloride, 4-chloro; Benzoyl chloride, p-chloro
- CAS RN.: 122-01-0
- EINECS: 204-515-3
- Molecular Weight: 175.0141
- Molecular Formula: C4H4Cl2O
- Density: 1.292g/cm3
- Melting Point(℃): 12-14℃
- Boiling Point(℃): 298.2°C at 760 mmHg
- Flash Point(℃): 162.3°C
- refractive_index: 1.643
- Hazard Symbols: C Details
- C:
- Risk Codes: R34; Details
- R34;: Details
- Safety Description: S26;S28A;Details
- S26;S28A;: Details
FAQ:
1. What is p-chlorobenzoyl chloride and what are its uses?
p-chlorobenzoyl chloride is a chemical compound that is commonly used in organic synthesis as a key intermediate in the production of pharmaceuticals, agrochemicals, and dyes. It is also utilized in the manufacturing of liquid crystal displays, polymers, and other specialty chemicals.
2. How is p-chlorobenzoyl chloride synthesized?
p-chlorobenzoyl chloride is typically synthesized through the reaction of p-chlorobenzoic acid with thionyl chloride, phosphorus pentachloride, or phosphorus oxychloride. This process involves the conversion of a carboxylic acid functional group into an acyl chloride, resulting in the formation of p-chlorobenzoyl chloride.
3. What are the key properties of p-chlorobenzoyl chloride?
p-chlorobenzoyl chloride is a white to pale yellow crystalline solid with a sharp, pungent odor. It is highly reactive and should be handled with caution due to its corrosive nature. The compound is sparingly soluble in water but readily dissolves in organic solvents such as diethyl ether, chloroform, and acetone.
4. What safety precautions should be taken when handling p-chlorobenzoyl chloride?
When working with p-chlorobenzoyl chloride, it is important to wear appropriate personal protective equipment, including gloves, safety goggles, and a lab coat. The compound should only be used in a well-ventilated area, and contact with skin, eyes, and mucous membranes should be avoided. In case of exposure, immediate medical attention is necessary.
5. Where can I purchase p-chlorobenzoyl chloride for my research or industrial needs?
p-chlorobenzoyl chloride is available for purchase from various chemical suppliers and distributors. It is important to source this compound from reputable suppliers who adhere to strict quality control standards and provide proper handling instructions. Ensure that you are compliant with all safety regulations and guidelines when purchasing and using p-chlorobenzoyl chloride.
p-chlorobenzoyl chloride is a chemical compound that is commonly used in organic synthesis as a key intermediate in the production of pharmaceuticals, agrochemicals, and dyes. It is also utilized in the manufacturing of liquid crystal displays, polymers, and other specialty chemicals.
2. How is p-chlorobenzoyl chloride synthesized?
p-chlorobenzoyl chloride is typically synthesized through the reaction of p-chlorobenzoic acid with thionyl chloride, phosphorus pentachloride, or phosphorus oxychloride. This process involves the conversion of a carboxylic acid functional group into an acyl chloride, resulting in the formation of p-chlorobenzoyl chloride.
3. What are the key properties of p-chlorobenzoyl chloride?
p-chlorobenzoyl chloride is a white to pale yellow crystalline solid with a sharp, pungent odor. It is highly reactive and should be handled with caution due to its corrosive nature. The compound is sparingly soluble in water but readily dissolves in organic solvents such as diethyl ether, chloroform, and acetone.
4. What safety precautions should be taken when handling p-chlorobenzoyl chloride?
When working with p-chlorobenzoyl chloride, it is important to wear appropriate personal protective equipment, including gloves, safety goggles, and a lab coat. The compound should only be used in a well-ventilated area, and contact with skin, eyes, and mucous membranes should be avoided. In case of exposure, immediate medical attention is necessary.
5. Where can I purchase p-chlorobenzoyl chloride for my research or industrial needs?
p-chlorobenzoyl chloride is available for purchase from various chemical suppliers and distributors. It is important to source this compound from reputable suppliers who adhere to strict quality control standards and provide proper handling instructions. Ensure that you are compliant with all safety regulations and guidelines when purchasing and using p-chlorobenzoyl chloride.

