
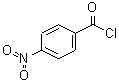
CAS: 122-04-3
p-nitrobenzoyl chloride
p-nitrobenzoyl chloride
Properties:
- Product Name: 4-Nitrobenzoyl chloride
- Synonyms:3355: 4-nitrobenzoic acid chloride; p-nitrobenzoyl chloride;
- CAS RN.: 122-04-3
- EINECS: 204-517-4
- Molecular Weight: 185.5646
- Molecular Formula: C7H4ClNO3
- Density: 1.453g/cm3
- Melting Point(℃): 71.5℃
- Boiling Point(℃): 277.8°C at 760 mmHg
- Flash Point(℃): 121.8°C
- refractive_index: 1.589
- Water Solubility: Decomposes
- Hazard Symbols: C Details
- C:
- Risk Codes: R34; Details
- R34;: Details
- Safety Description: S26;S36/37/39;S45;Details
- S26;S36/37/39;S45;: Details
FAQ:
What is p-nitrobenzoyl chloride and how is it used in various
industries?
p-nitrobenzoyl chloride, also known as 4-nitrobenzoyl chloride or 4-chloro-3-nitrobenzoyl chloride, is a valuable chemical intermediate used in the synthesis of pharmaceuticals, agrochemicals, dyes, and other organic compounds. It is commonly utilized as an acylating agent in the production of esters and amides, as well as a key building block in the preparation of various complex molecules. The high reactivity of p-nitrobenzoyl chloride makes it a versatile compound with wide-ranging applications in the chemical industry.
How is p-nitrobenzoyl chloride synthesized and what are the key properties of this compound?
p-nitrobenzoyl chloride is typically synthesized by the reaction of p-nitrobenzoic acid with thionyl chloride or phosphorus pentachloride under reflux conditions. The resulting compound is a yellow crystalline solid with a melting point of around 75-77°C and a boiling point of approximately 144-146°C at atmospheric pressure. p-nitrobenzoyl chloride is soluble in a variety of organic solvents such as dichloromethane, ethyl acetate, and acetonitrile, making it suitable for various chemical reactions and processes.
What are the main applications of p-nitrobenzoyl chloride in the pharmaceutical industry?
p-nitrobenzoyl chloride is widely used in the pharmaceutical industry for the synthesis of various active pharmaceutical ingredients (APIs) and drug intermediates. It can serve as a key starting material for the preparation of pharmaceutical compounds with diverse therapeutic properties, including anti-inflammatory, antiviral, and antibacterial activities. p-nitrobenzoyl chloride is particularly valuable in the pharmaceutical sector due to its ability to modify the chemical structure of organic molecules and enhance their biological activity.
How does p-nitrobenzoyl chloride contribute to the production of agrochemicals and crop protection products?
p-nitrobenzoyl chloride plays a crucial role in the synthesis of agrochemicals and crop protection products by enabling the efficient modification of pesticide molecules. By acylating or amidating specific functional groups in organic compounds, p-nitrobenzoyl chloride facilitates the development of novel agrochemicals with enhanced pesticidal activity, selectivity, and stability. Its reactivity and versatility make it a valuable tool for the design and synthesis of next-generation crop protection solutions that support sustainable agriculture and environmental stewardship.
What are the environmental and safety considerations associated with the handling and storage of p-nitrobenzoyl chloride?
p-nitrobenzoyl chloride is a hazardous chemical that should be handled with care to minimize risks to human health and the environment. It is essential to use appropriate personal protective equipment (PPE) such as gloves, goggles, and lab coats when working with p-nitrobenzoyl chloride to prevent direct contact with the skin, eyes, or mucous membranes. Furthermore, p-nitrobenzoyl chloride should be stored in a cool, dry, well-ventilated area away from sources of heat, light, and moisture to avoid decomposition or spontaneous ignition. Proper safety protocols and containment measures should be implemented to prevent spills, leaks, or exposure to this reactive compound.
p-nitrobenzoyl chloride, also known as 4-nitrobenzoyl chloride or 4-chloro-3-nitrobenzoyl chloride, is a valuable chemical intermediate used in the synthesis of pharmaceuticals, agrochemicals, dyes, and other organic compounds. It is commonly utilized as an acylating agent in the production of esters and amides, as well as a key building block in the preparation of various complex molecules. The high reactivity of p-nitrobenzoyl chloride makes it a versatile compound with wide-ranging applications in the chemical industry.
How is p-nitrobenzoyl chloride synthesized and what are the key properties of this compound?
p-nitrobenzoyl chloride is typically synthesized by the reaction of p-nitrobenzoic acid with thionyl chloride or phosphorus pentachloride under reflux conditions. The resulting compound is a yellow crystalline solid with a melting point of around 75-77°C and a boiling point of approximately 144-146°C at atmospheric pressure. p-nitrobenzoyl chloride is soluble in a variety of organic solvents such as dichloromethane, ethyl acetate, and acetonitrile, making it suitable for various chemical reactions and processes.
What are the main applications of p-nitrobenzoyl chloride in the pharmaceutical industry?
p-nitrobenzoyl chloride is widely used in the pharmaceutical industry for the synthesis of various active pharmaceutical ingredients (APIs) and drug intermediates. It can serve as a key starting material for the preparation of pharmaceutical compounds with diverse therapeutic properties, including anti-inflammatory, antiviral, and antibacterial activities. p-nitrobenzoyl chloride is particularly valuable in the pharmaceutical sector due to its ability to modify the chemical structure of organic molecules and enhance their biological activity.
How does p-nitrobenzoyl chloride contribute to the production of agrochemicals and crop protection products?
p-nitrobenzoyl chloride plays a crucial role in the synthesis of agrochemicals and crop protection products by enabling the efficient modification of pesticide molecules. By acylating or amidating specific functional groups in organic compounds, p-nitrobenzoyl chloride facilitates the development of novel agrochemicals with enhanced pesticidal activity, selectivity, and stability. Its reactivity and versatility make it a valuable tool for the design and synthesis of next-generation crop protection solutions that support sustainable agriculture and environmental stewardship.
What are the environmental and safety considerations associated with the handling and storage of p-nitrobenzoyl chloride?
p-nitrobenzoyl chloride is a hazardous chemical that should be handled with care to minimize risks to human health and the environment. It is essential to use appropriate personal protective equipment (PPE) such as gloves, goggles, and lab coats when working with p-nitrobenzoyl chloride to prevent direct contact with the skin, eyes, or mucous membranes. Furthermore, p-nitrobenzoyl chloride should be stored in a cool, dry, well-ventilated area away from sources of heat, light, and moisture to avoid decomposition or spontaneous ignition. Proper safety protocols and containment measures should be implemented to prevent spills, leaks, or exposure to this reactive compound.

