
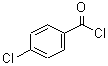
CAS: 122-01-0
parachlorobenzoyl chloride
parachlorobenzoyl chloride
Properties:
- Product Name: 4-Chlorobenzoyl chloride
- Synonyms:3331: PCOC; p-Chlorobenzolychloride; 4-Chlorobenzolychloride; p-chlorlbenzoyl chloride; p-chlorobenzoyl chloride; parachlorobenzoyl chloride; 4-chloro-6-methylquinazoline; Benzoyl chloride, 4-chloro; Benzoyl chloride, p-chloro
- CAS RN.: 122-01-0
- EINECS: 204-515-3
- Molecular Weight: 175.0141
- Molecular Formula: C4H4Cl2O
- Density: 1.292g/cm3
- Melting Point(℃): 12-14℃
- Boiling Point(℃): 298.2°C at 760 mmHg
- Flash Point(℃): 162.3°C
- refractive_index: 1.643
- Hazard Symbols: C Details
- C:
- Risk Codes: R34; Details
- R34;: Details
- Safety Description: S26;S28A;Details
- S26;S28A;: Details
FAQ:
What is parachlorobenzoyl chloride and what is it used for?
Parachlorobenzoyl chloride is a chemical compound that is commonly used as an intermediate in the production of various organic compounds, including pharmaceuticals, dyes, and agrochemicals. It is often utilized in the synthesis of other chemicals due to its versatile reactivity and functional groups.
What are the main applications of parachlorobenzoyl chloride?
Parachlorobenzoyl chloride is primarily used in the pharmaceutical industry for the production of active pharmaceutical ingredients (APIs) and intermediates. It is also used in the production of dyes, agrochemicals, and other specialty chemicals. Its high reactivity and versatility make it a valuable building block in organic synthesis.
What are the key properties of parachlorobenzoyl chloride?
Parachlorobenzoyl chloride is a colorless to pale yellow liquid with a strong, irritating odor. It is soluble in most organic solvents and reacts readily with water and alcohols. It is highly reactive due to the presence of the chloro and benzoyl functional groups, which make it an excellent acylating agent in various chemical reactions.
How is parachlorobenzoyl chloride synthesized?
Parachlorobenzoyl chloride is typically synthesized through the reaction of parachlorobenzoic acid with thionyl chloride or phosphorus pentachloride. The reaction typically occurs under anhydrous conditions and at elevated temperatures to promote the conversion of the acid to the corresponding acid chloride. The resulting product is then purified through distillation or other separation techniques.
What are the safety considerations when handling parachlorobenzoyl chloride?
Parachlorobenzoyl chloride is a highly reactive and irritating compound that should be handled with caution. It can cause skin and eye irritation upon contact and should be used in a well-ventilated area to prevent exposure to fumes. Proper personal protective equipment, such as gloves and goggles, should be worn when handling this compound to minimize the risk of exposure. Additionally, spillages should be cleaned up promptly and waste disposal should be done in accordance with local regulations.
Parachlorobenzoyl chloride is a chemical compound that is commonly used as an intermediate in the production of various organic compounds, including pharmaceuticals, dyes, and agrochemicals. It is often utilized in the synthesis of other chemicals due to its versatile reactivity and functional groups.
What are the main applications of parachlorobenzoyl chloride?
Parachlorobenzoyl chloride is primarily used in the pharmaceutical industry for the production of active pharmaceutical ingredients (APIs) and intermediates. It is also used in the production of dyes, agrochemicals, and other specialty chemicals. Its high reactivity and versatility make it a valuable building block in organic synthesis.
What are the key properties of parachlorobenzoyl chloride?
Parachlorobenzoyl chloride is a colorless to pale yellow liquid with a strong, irritating odor. It is soluble in most organic solvents and reacts readily with water and alcohols. It is highly reactive due to the presence of the chloro and benzoyl functional groups, which make it an excellent acylating agent in various chemical reactions.
How is parachlorobenzoyl chloride synthesized?
Parachlorobenzoyl chloride is typically synthesized through the reaction of parachlorobenzoic acid with thionyl chloride or phosphorus pentachloride. The reaction typically occurs under anhydrous conditions and at elevated temperatures to promote the conversion of the acid to the corresponding acid chloride. The resulting product is then purified through distillation or other separation techniques.
What are the safety considerations when handling parachlorobenzoyl chloride?
Parachlorobenzoyl chloride is a highly reactive and irritating compound that should be handled with caution. It can cause skin and eye irritation upon contact and should be used in a well-ventilated area to prevent exposure to fumes. Proper personal protective equipment, such as gloves and goggles, should be worn when handling this compound to minimize the risk of exposure. Additionally, spillages should be cleaned up promptly and waste disposal should be done in accordance with local regulations.

