
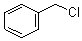
CAS: 100-44-7
DBU/benzyl chloride
DBU/benzyl chloride
Properties:
- Product Name: Benzyl Chloride
- Synonyms:5464: α-Chlorotoluene; ω-Chlorotoluene; 1-Chlorome-thylbenzene; (Chloromethyl)Benzene; Tolyl chloride; DBU/benzyl chloride; A-CHLOROTOLUENE; ALPHA-CHLOROTOLUENE; AKOS BBS-00003953; (chloromethyl)-benzen; (chloromethyl)-Benzene; 1-Chloromethylbenzene; ai3-15518; alpha-chloro-toluen; alpha-Chlortoluol; alpha-tolylchloride
- CAS RN.: 100-44-7
- EINECS: 202-853-6
- Molecular Weight: 126.5835
- Molecular Formula: C7H7Cl
- Density: 1.08g/cm3
- Melting Point(℃): -39℃
- Boiling Point(℃): 179.4°C at 760 mmHg
- Flash Point(℃): 73.9°C
- refractive_index: 1.527
- Water Solubility: 0.3 g/L (20℃)
- Hazard Symbols: T Details
- T:
- Risk Codes: R22;R23;R37/38;R41;R45;R48/22; Details
- R22;R23;R37/38;R41;R45;R48/22;: Details
- Safety Description: S45;S53;Details
- S45;S53;: Details
FAQ:
What is DBU/benzyl chloride and how is it used?
DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) is a strong, non-nucleophilic base commonly used in organic synthesis. Benzyl chloride is an organic compound used as a reagent in the manufacture of various pharmaceuticals, dyes, and perfumes. When combined, DBU/benzyl chloride serves as a key intermediate in the synthesis of a wide range of organic compounds.
What are the benefits of using DBU/benzyl chloride in organic synthesis?
DBU/benzyl chloride offers several advantages in organic synthesis. The combination of DBU and benzyl chloride allows for efficient and selective formation of a variety of chemical bonds. Additionally, the use of DBU/benzyl chloride often results in higher yields and fewer byproducts compared to other methods. This versatile reagent combination is particularly useful in the construction of complex molecules.
How is DBU/benzyl chloride different from other reagents commonly used in organic synthesis?
DBU/benzyl chloride differs from other reagents commonly used in organic synthesis in several ways. Unlike many nucleophilic bases, DBU is non-nucleophilic, making it an excellent choice for reactions where unwanted side reactions may occur. Additionally, the combination of DBU and benzyl chloride offers unique reactivity that is not easily achieved with other reagents. This makes DBU/benzyl chloride a valuable tool for chemists working on challenging organic synthesis projects.
What are some common reactions that can be carried out using DBU/benzyl chloride?
DBU/benzyl chloride can be used in a variety of reactions, including nucleophilic substitution, dehydrohalogenation, and formation of carbon-carbon bonds. The reagent combination is particularly well-suited for the synthesis of amino acids, peptides, and other biologically active compounds. It is also useful in the preparation of heterocyclic compounds and other complex organic molecules. The versatility of DBU/benzyl chloride makes it a valuable addition to any organic chemist's toolkit.
Are there any precautions to consider when working with DBU/benzyl chloride?
While DBU/benzyl chloride is a valuable reagent in organic synthesis, it is important to handle it with care. Both DBU and benzyl chloride can be irritating to the skin, eyes, and respiratory tract, so appropriate personal protective equipment should be worn when working with these compounds. Additionally, DBU is sensitive to moisture and should be stored in a dry environment to prevent degradation. Proper handling and storage of DBU/benzyl chloride will help ensure the safety of the operator and the success of the synthesis reactions.
DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) is a strong, non-nucleophilic base commonly used in organic synthesis. Benzyl chloride is an organic compound used as a reagent in the manufacture of various pharmaceuticals, dyes, and perfumes. When combined, DBU/benzyl chloride serves as a key intermediate in the synthesis of a wide range of organic compounds.
What are the benefits of using DBU/benzyl chloride in organic synthesis?
DBU/benzyl chloride offers several advantages in organic synthesis. The combination of DBU and benzyl chloride allows for efficient and selective formation of a variety of chemical bonds. Additionally, the use of DBU/benzyl chloride often results in higher yields and fewer byproducts compared to other methods. This versatile reagent combination is particularly useful in the construction of complex molecules.
How is DBU/benzyl chloride different from other reagents commonly used in organic synthesis?
DBU/benzyl chloride differs from other reagents commonly used in organic synthesis in several ways. Unlike many nucleophilic bases, DBU is non-nucleophilic, making it an excellent choice for reactions where unwanted side reactions may occur. Additionally, the combination of DBU and benzyl chloride offers unique reactivity that is not easily achieved with other reagents. This makes DBU/benzyl chloride a valuable tool for chemists working on challenging organic synthesis projects.
What are some common reactions that can be carried out using DBU/benzyl chloride?
DBU/benzyl chloride can be used in a variety of reactions, including nucleophilic substitution, dehydrohalogenation, and formation of carbon-carbon bonds. The reagent combination is particularly well-suited for the synthesis of amino acids, peptides, and other biologically active compounds. It is also useful in the preparation of heterocyclic compounds and other complex organic molecules. The versatility of DBU/benzyl chloride makes it a valuable addition to any organic chemist's toolkit.
Are there any precautions to consider when working with DBU/benzyl chloride?
While DBU/benzyl chloride is a valuable reagent in organic synthesis, it is important to handle it with care. Both DBU and benzyl chloride can be irritating to the skin, eyes, and respiratory tract, so appropriate personal protective equipment should be worn when working with these compounds. Additionally, DBU is sensitive to moisture and should be stored in a dry environment to prevent degradation. Proper handling and storage of DBU/benzyl chloride will help ensure the safety of the operator and the success of the synthesis reactions.

