
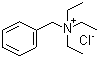
CAS: 56-37-1
benzyltrietylammonium chloride
benzyltrietylammonium chloride
Properties:
- Product Name: Benzyl Triethyl Ammonium Chloride
- Synonyms:93939: teba; tebac; benzyl triethylammonium chloride; triethyl benzyl ammonium chloride; benzyltrietylammonium chloride; benzyltriethylammonium chloride; bteac; n,n,n'-triethylbenzenemethanaminium chloride; n,n,n-triethyl-benzenemethanaminium chloride; tri ethyl benzyl ammonium chloride; Benzyl tri ethyl ammonium chloride; N-benzyl-N,N-diethylethanaminium chloride; Benzyltriethylammoniumchloride
- CAS RN.: 56-37-1
- EINECS: 200-270-1
- Molecular Weight: 227.7735
- Molecular Formula: C13H22ClN
- Melting Point(℃): 185℃
- Water Solubility: 700 g/L (20℃)
- Hazard Symbols: Xi Details
- Xi:
- Risk Codes: R36/37/38; Details
- R36/37/38;: Details
- Safety Description: S26;S37/39;Details
- S26;S37/39;: Details
FAQ:
What is benzyltriethylammonium chloride and what is its role in chemistry
applications?
Benzyltriethylammonium chloride is a quaternary ammonium salt that is commonly used in various chemical reactions in both academic and industrial settings. It serves as a phase-transfer catalyst, aiding in the transfer of ions between immiscible phases. This compound is particularly useful in organic synthesis, where it helps to facilitate reactions that would otherwise be challenging to accomplish. Benzyltriethylammonium chloride plays a crucial role in enabling the synthesis of a wide range of organic compounds.
How does benzyltriethylammonium chloride function as a phase-transfer catalyst?
Benzyltriethylammonium chloride functions as a phase-transfer catalyst by forming a complex with the reactants in a reaction mixture. This complex can readily move between the two immiscible phases, such as between an aqueous phase and an organic phase. The catalyst facilitates the transfer of ions or molecules between these phases, allowing for reactions to proceed smoothly. Benzyltriethylammonium chloride acts as a mediator, promoting interactions between the reactants that would otherwise be hindered by the immiscibility of the phases.
What are some common reactions that utilize benzyltriethylammonium chloride as a catalyst?
Benzyltriethylammonium chloride is commonly employed as a catalyst in reactions such as nucleophilic substitutions, oxidations, alkylations, and esterifications. For example, it can assist in the alkylation of phenols, the synthesis of quaternary ammonium salts, and the preparation of esters from carboxylic acids and alcohols. The versatility of benzyltriethylammonium chloride as a phase-transfer catalyst makes it a valuable tool in organic synthesis for a variety of applications.
How does benzyltriethylammonium chloride compare to other phase-transfer catalysts on the market?
Benzyltriethylammonium chloride offers several advantages over other phase-transfer catalysts, including its high efficiency, ease of handling, and compatibility with a wide range of reactions. Its quaternary ammonium structure imparts stability and reactivity, making it a reliable catalyst for various chemical transformations. Additionally, benzyltriethylammonium chloride is cost-effective and readily available, making it a preferred choice for many researchers and chemists. Overall, benzyltriethylammonium chloride stands out for its effectiveness and versatility as a phase-transfer catalyst.
How can researchers and chemists best utilize benzyltriethylammonium chloride in their work?
Researchers and chemists can maximize the benefits of benzyltriethylammonium chloride by carefully selecting appropriate reaction conditions and concentrations. It is essential to optimize the catalyst loading and reaction parameters to achieve the desired outcomes efficiently. Additionally, proper storage and handling of benzyltriethylammonium chloride are crucial to maintaining its effectiveness over time. By following best practices and understanding the unique properties of this compound, researchers can leverage benzyltriethylammonium chloride to advance their research and achieve successful outcomes in various chemical reactions.
Benzyltriethylammonium chloride is a quaternary ammonium salt that is commonly used in various chemical reactions in both academic and industrial settings. It serves as a phase-transfer catalyst, aiding in the transfer of ions between immiscible phases. This compound is particularly useful in organic synthesis, where it helps to facilitate reactions that would otherwise be challenging to accomplish. Benzyltriethylammonium chloride plays a crucial role in enabling the synthesis of a wide range of organic compounds.
How does benzyltriethylammonium chloride function as a phase-transfer catalyst?
Benzyltriethylammonium chloride functions as a phase-transfer catalyst by forming a complex with the reactants in a reaction mixture. This complex can readily move between the two immiscible phases, such as between an aqueous phase and an organic phase. The catalyst facilitates the transfer of ions or molecules between these phases, allowing for reactions to proceed smoothly. Benzyltriethylammonium chloride acts as a mediator, promoting interactions between the reactants that would otherwise be hindered by the immiscibility of the phases.
What are some common reactions that utilize benzyltriethylammonium chloride as a catalyst?
Benzyltriethylammonium chloride is commonly employed as a catalyst in reactions such as nucleophilic substitutions, oxidations, alkylations, and esterifications. For example, it can assist in the alkylation of phenols, the synthesis of quaternary ammonium salts, and the preparation of esters from carboxylic acids and alcohols. The versatility of benzyltriethylammonium chloride as a phase-transfer catalyst makes it a valuable tool in organic synthesis for a variety of applications.
How does benzyltriethylammonium chloride compare to other phase-transfer catalysts on the market?
Benzyltriethylammonium chloride offers several advantages over other phase-transfer catalysts, including its high efficiency, ease of handling, and compatibility with a wide range of reactions. Its quaternary ammonium structure imparts stability and reactivity, making it a reliable catalyst for various chemical transformations. Additionally, benzyltriethylammonium chloride is cost-effective and readily available, making it a preferred choice for many researchers and chemists. Overall, benzyltriethylammonium chloride stands out for its effectiveness and versatility as a phase-transfer catalyst.
How can researchers and chemists best utilize benzyltriethylammonium chloride in their work?
Researchers and chemists can maximize the benefits of benzyltriethylammonium chloride by carefully selecting appropriate reaction conditions and concentrations. It is essential to optimize the catalyst loading and reaction parameters to achieve the desired outcomes efficiently. Additionally, proper storage and handling of benzyltriethylammonium chloride are crucial to maintaining its effectiveness over time. By following best practices and understanding the unique properties of this compound, researchers can leverage benzyltriethylammonium chloride to advance their research and achieve successful outcomes in various chemical reactions.

