
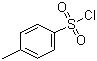
CAS: 98-59-9
p-Toluene Benzyl Sulfonyl Chloride
p-Toluene Benzyl Sulfonyl Chloride
Properties:
- Product Name: Tosyl chloride
- Synonyms:194508: 4-Toluene sulfochloride; 4-Methylbenzenesulfonyl chloride;p-Toluenesulfonyl chloride; Toluene-4-sulfonyl chloride; 4-Toluolsulfonyl chloride; p-Tosyl Chloride; PTSC; P-toluene sulfonyl chloride; p-Toluene Benzyl Sulfonyl Chloride; 4-toluene sulfonyl chloride; P-toluene Sulphonyl chloride; 4-Toluenesulfonyl chloride
- CAS RN.: 98-59-9
- EINECS: 202-684-8
- Molecular Weight: 190.6473
- Molecular Formula: C7H7ClO2S
- Density: 1.339g/cm3
- Melting Point(℃): 67-69℃
- Boiling Point(℃): 265.3°C at 760 mmHg
- Flash Point(℃): 114.3°C
- refractive_index: 1.545
- Water Solubility: hydrolyses
- Hazard Symbols: C Details
- C:
- Risk Codes: R29;R34; Details
- R29;R34;: Details
- Safety Description: S26;S36/37/39;S45;Details
- S26;S36/37/39;S45;: Details
FAQ:
What is p-Toluene Benzyl Sulfonyl Chloride used for?
p-Toluene Benzyl Sulfonyl Chloride is commonly used as a reagent in organic synthesis for the protection of alcohols and amines.
How does p-Toluene Benzyl Sulfonyl Chloride work as a protecting group?
p-Toluene Benzyl Sulfonyl Chloride reacts with alcohols and amines to form stable sulfonyl derivatives, providing protection against unwanted reactions in organic synthesis.
What are some common reactions where p-Toluene Benzyl Sulfonyl Chloride is used?
p-Toluene Benzyl Sulfonyl Chloride is commonly used in reactions such as protection of hydroxyl groups in carbohydrates, amino acid derivatives, and peptides, as well as in the synthesis of pharmaceutical compounds.
How is p-Toluene Benzyl Sulfonyl Chloride different from other protecting groups?
p-Toluene Benzyl Sulfonyl Chloride is known for its stability under various reaction conditions, making it a versatile and reliable choice for protecting alcohols and amines in organic synthesis.
What are the key benefits of using p-Toluene Benzyl Sulfonyl Chloride in organic synthesis?
p-Toluene Benzyl Sulfonyl Chloride offers high selectivity in protecting specific functional groups, excellent stability during various reaction conditions, and easy deprotection to regenerate the original functional group.
p-Toluene Benzyl Sulfonyl Chloride is commonly used as a reagent in organic synthesis for the protection of alcohols and amines.
How does p-Toluene Benzyl Sulfonyl Chloride work as a protecting group?
p-Toluene Benzyl Sulfonyl Chloride reacts with alcohols and amines to form stable sulfonyl derivatives, providing protection against unwanted reactions in organic synthesis.
What are some common reactions where p-Toluene Benzyl Sulfonyl Chloride is used?
p-Toluene Benzyl Sulfonyl Chloride is commonly used in reactions such as protection of hydroxyl groups in carbohydrates, amino acid derivatives, and peptides, as well as in the synthesis of pharmaceutical compounds.
How is p-Toluene Benzyl Sulfonyl Chloride different from other protecting groups?
p-Toluene Benzyl Sulfonyl Chloride is known for its stability under various reaction conditions, making it a versatile and reliable choice for protecting alcohols and amines in organic synthesis.
What are the key benefits of using p-Toluene Benzyl Sulfonyl Chloride in organic synthesis?
p-Toluene Benzyl Sulfonyl Chloride offers high selectivity in protecting specific functional groups, excellent stability during various reaction conditions, and easy deprotection to regenerate the original functional group.

